National HIV treatment programs are preparing for big shifts in their national treatment guidelines and patient regimens. The World Health Organization’s (WHO) 2016 guidelines included a number of antiretroviral (ARV) medications that are more effective, tolerable and affordable (some of which are now available), in addition to optimal pediatric formulations which improve upon limited treatment options for children. Accelerating access to the best available treatment options not only better serves the needs of patients, but also supports significant scale up of treatment, saves money, and achieves public health goals, including reducing rates of new infections and improving survival rates in the long-term.
Making new products widely accessible to patients who can benefit from them requires high levels of planning, coordination and targeted implementation. With support from Unitaid, the Clinton Health Access Initiative’s (CHAI) HIV Access Program developed an online HIV New Product Introduction Toolkit to provide tools and resources to assist national programs, partners and community representatives as they evaluate, plan and execute transitions to new ARVs in their local context.
The Toolkit leverages expertise developed through CHAI’s long-term partnerships with Ministries of Health on new product introduction. It guides the user through the various stages of new product introduction, from adoption to uptake monitoring, ensuring that products reach patients in need.
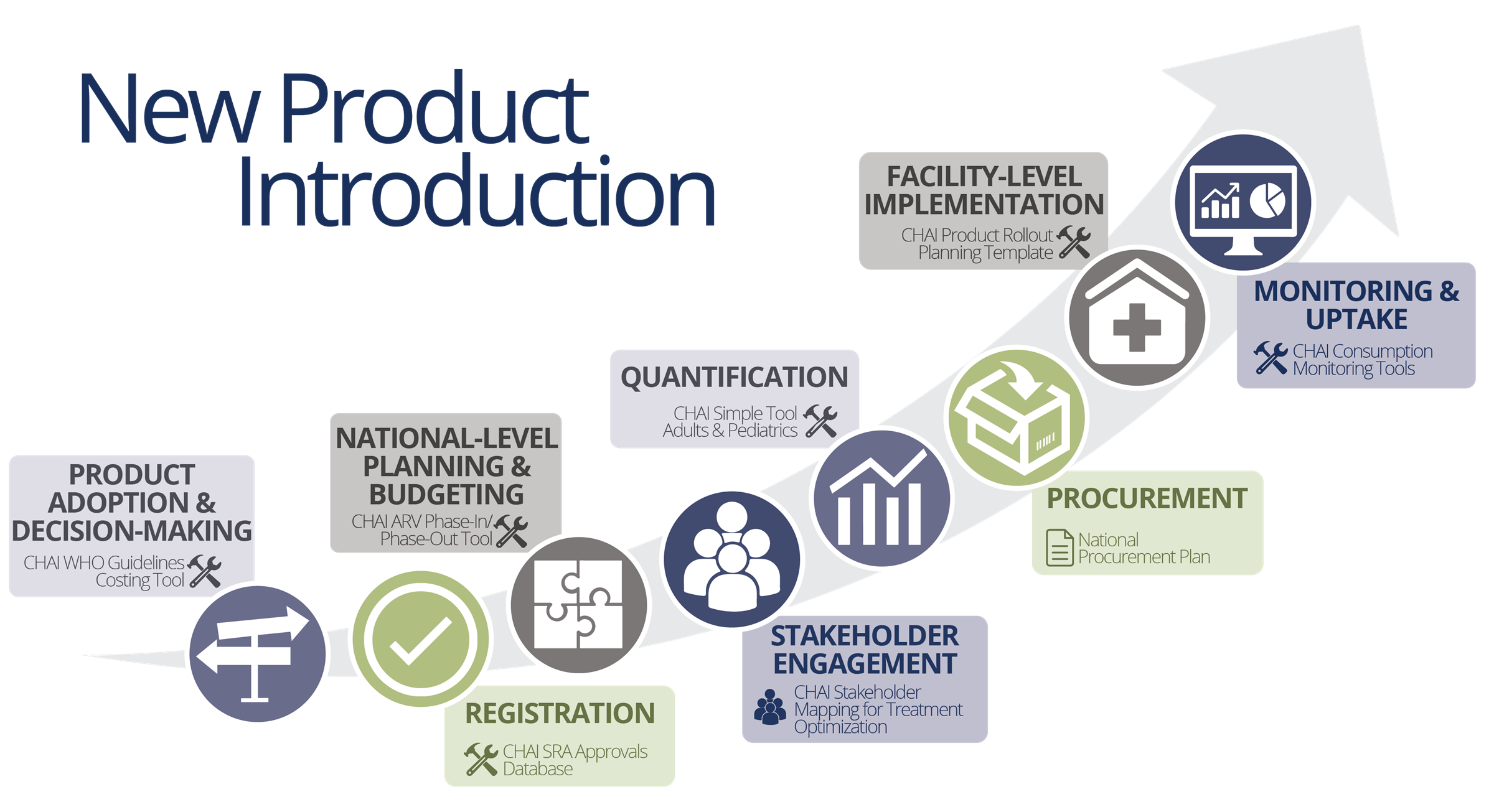
Key tools and resources included across each step in the Toolkit include:
- CHAI WHO Guidelines Costing Tool to assist in guidelines adoption decision-making and assessing budgetary implications of new product use
- CHAI ARV Phase-In/Phase-Out Tool to plan rollout strategies based on priority populations, region or facility type for new product Tenofovir/Lamivudine/Dolutegravir
- CHAI Simple Tool for forecasting to inform procurement and supply planning
- Community Resources for civil society organizations involved in the product rollout process
- CHAI Rapid Uptake Monitoring Tool to rapidly compare consumption against planned uptake during the early stages of rollout to avoid stock-outs and wastage
In addition, the Toolkit serves as a repository for new product introduction resources from a wide range of partners including the ARV Procurement Working Group (APWG), HIV i-Base, AfroCAB, Inter-Agency Task Team on Children and HIV and AIDS (IATT), Drugs for Neglected Diseases initiative (DNDi), the U.S. Agency for International Development (USAID), among others. The toolkit is designed to evolve based on contributions from all involved as new experiences, best practices, and lessons learned are generated and new or updated tools and resources are developed across Ministries of Health and partners.
For more information on the HIV New Product Introduction Toolkit or HIV New Product Introduction Guide, or to contribute resources and tools, contact: HIVToolkit@clintonhealthaccess.org.





