As we celebrate World Immunization Week, it is time to reflect on the powerful success of the polio vaccine and the importance of ensuring that all children have access to lifesaving vaccinations around the globe.
Poliomyelitis (also known as polio) is a highly infectious viral disease, which mainly affects children under 5 years of age. The virus is transmitted by the fecal-oral route and by aerosol droplets. It multiplies in the intestine, where it can invade the nervous system and cause irreversible paralysis. Polio was the leading cause of lower limb paralysis in the pre-vaccine era, crippling over 350,000 children annually.
In 1988, the World Health Assembly adopted a resolution for the worldwide eradication of polio, and launched the Global Polio Eradication Initiative (GPEI). Since then, the number of polio cases has decreased by over 99%, falling to just 74 cases in 2015. Today, more than 15 million people are able to walk who would have otherwise been paralyzed.
These successes have largely been attributed to the use of the trivalent oral polio vaccine (tOPV), which contains all 3 serotypes (1, 2, 3) of attenuated (weakened) polioviruses. Indeed, the use of this vaccine led to the worldwide eradication of wild poliovirus type 2, with the last case occurring in 1999. The use of tOPV, however, has on rare occasions resulted in vaccine-associated paralytic polio (VAPP) and circulating vaccine-derived poliovirus (cVDPV). Reports in 2016 indicated that over 90% of cVDPV cases and approximately 40% of VAPP cases were due to the type 2 component of tOPV. Given the risk the type 2 component of tOPV potentially poses to a world free of polio virus type 2, the GPEI recommended countries still using tOPV in their the routine immunization programs to switch to bivalent OPV (bOPV), containing just type 1 and 3 polioviruses. Removal of the type component of the tOPV, which is also known to interfere with the immune responses to poliovirus types 1 and 3, may also help to galvanize polio eradication efforts.
In Cameroon, like in other parts of the world, tremendous strides have been made toward polio eradication. In 2009, the country was declared free of wild polio. This declaration, however, was short-lived as 7 new cases of polioviruses were detected between October 2013 and April 2014. Three of these cases were due to wild poliovirus type 1, while the remaining 4 were due to cVDPV. These cases prompted the World Health Organization (WHO) to declare the country a polio-prone nation (polio exporter country). In response to this outbreak, the Ministry of Health (MOH) conducted multiple rounds of polio vaccination campaigns, using the trivalent Oral Polio vaccines (tOPV). Thanks to these campaigns, Cameroon was declared polio-free in April 2015.
In September 2015, Cameroon adhered to the the GPEI recommendation, to switch from tOPV to to bivalent OPV (bOPV), so as to eliminate the rare risk of cVDPV and VAPP. Globally, the switch was planned to occur synchronously in the 156 countries and territories still using tOPV between 17th April and 1st May 2016, and represented the first of its kind in the history of global health.
Consequently, its success depended on careful and detailed planning, preparation and execution. The Clinton Health Access Initiative (CHAI), with generous support from the Bill and Melinda Gates Foundation and in partnership with the MOH, UNICEF and the WHO, played a key role in each of these phases, including supporting execution of the switch in countries where CHAI works.
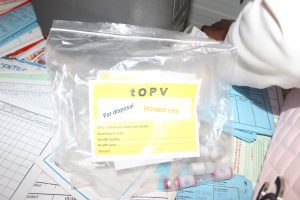
In Cameroon, CHAI supported the MOH to make the switch on April 20, 2016; in a single day tOPV was to be removed from roughly 4,500 health facilities and other vaccines storage sites (including central, regional, and district warehouses, as well as laboratories) and to be replaced with bOPV. Cameroon’s Minister of Public Health established a technical working group, which was led by the Expanded Program on Immunization (EPI) with the support from its partners, to coordinate the overall switch activities. CHAI and partners supported the EPI to prepare clear terms of references for the different committees of the technical working group as well as a detailed workplan for the operations.
The first major task of the technical working group was to develop a costed switch plan, which was approved by the GPEI in November 2015 making Cameroon among the first few countries with a GPEI-approved switch plan. Through this approval, Cameroon secured 50% of the estimated US$600,000 switch budget from the GPEI. One of the biggest challenges for this operation was to close the remaining funding gap given that the activity was occurring outside the government’s budgeting cycle.
To address this challenge, CHAI worked closely with the MOH and partners to identify the best budget-closing approach, partnering the switch with an already funded activity: African Vaccination Week. This synergy addressed all of the training costs associate with the switch. CHAI took the lead in drafting the training modules and organized the production and distribution of tOPV withdrawal materials.
In addition, CHAI supported the EPI in planning and conducting nationwide tOPV inventories. This enabled the country to accurately assess the level of in-country stocks and adjust the forecast for upcoming orders of tOPV, which in turn led to the importation and distribution of only the required quantity of tOPV.
Given the complexity of the switch, CHAI organized a dry-run in three health districts to identify potential bottlenecks that could hinder smooth implementation. The dry-run enabled the EPI to update the training modules and strategy for tOPV withdrawal and bOPV distribution, particularly to health areas without functional vaccine refrigerators. CHAI also supported the EPI to incorporate the lessons learned from this simulation into national and regional operational workplans. These lessons had impact beyond Cameroon as CHAI was able to share them with other countries in the Central and West African sub-region.
The dry-run unveiled the critical need for Cameroon to quickly develop strong coordination and management of the tOPV once it had been withdrawn from health facilities. CHAI took the lead in developing detailed operational plans and coordinated the shipment of withdrawn tOPV from the regions to an accredited site to ensure destruction in accordance with international standards in an environmentally sound manner.
The extensive coordination toward the withdrawal and elimination of tOPV led to the WHO making Cameroon one of the first six countries in the world to obtain switch validation on May 4, 2016. This has eliminated the risk of exposure to type 2-induced poliomyelitis.
The OPV switch highlighted the impact that robust and rigorous planning and coordination can have on the implementation of complex public health projects. We hope that these lessons will be leveraged in the process of eradication of polio, namely the total withdrawal of bOPV from routine immunization expected to occur in 2019.





